How Is The Template Strand Determined In Repair
What is CRISPR?
What is CRISPR?
CRISPR is a powerful tool for editing genomes, pregnant it allows researchers to easily alter DNA sequences and modify factor function. It has many potential applications, including correcting genetic defects, treating and preventing the spread of diseases, and improving the growth and resilience of crops. Still, despite its promise, the technology also raises upstanding concerns.
In popular usage, "CRISPR" (pronounced "crisper") is shorthand for "CRISPR-Cas9." CRISPRs are specialized stretches of Deoxyribonucleic acid, and the protein Cas9 — where Cas stands for "CRISPR-associated" — is an enzyme that acts like a pair of molecular scissors, capable of cutting strands of Dna.
CRISPR engineering science was adapted from the natural defense mechanisms of bacteria and archaea, a domain of relatively simple single-celled microorganisms. These organisms use CRISPR-derived RNA, a molecular cousin to Deoxyribonucleic acid, and diverse Cas proteins to foil attacks by viruses. To foil attacks, the organisms chop up the DNA of viruses and and then stow bits of that DNA in their own genome, to exist used equally a weapon against the foreign invaders should those viruses attack once more.
When the components of CRISPR are transferred into other, more circuitous, organisms, those components tin so manipulate genes, a process called "gene editing." No one really knew what this process looked like until 2022, when a team of researchers led by Mikihiro Shibata of Kanazawa University in Nihon and Hiroshi Nishimasu of the Academy of Tokyo showed, for the very first time, what it looks like when a CRISPR is in action, Alive Science previously reported.
Related: Genetics by the numbers: 10 tantalizing tales
Key components of CRISPR
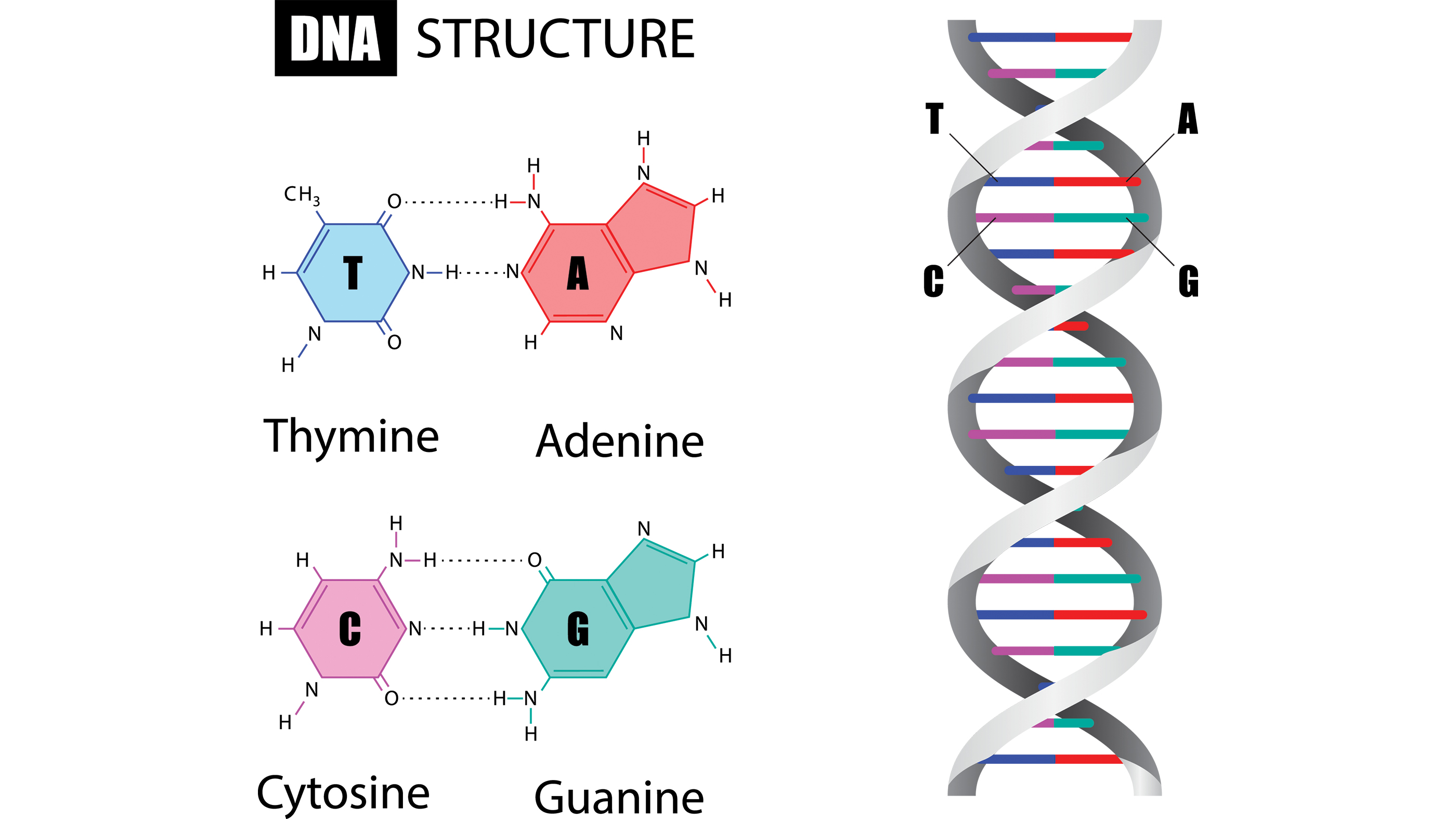
CRISPRs: The term "CRISPR" stands for "clusters of regularly interspaced brusque palindromic repeats" and describes a region of DNA fabricated upwards of short, repeated sequences with and so-called "spacers" sandwiched between each repeat.
When we talk about repeats in the genetic code, nosotros're talking well-nigh the ordering of rungs within the spiral ladder of a Deoxyribonucleic acid molecule. Each rung contains ii chemic bases bound together: A base called adenine (A) links upward to another called thymine (T), and the base guanine (G) pairs with cytosine (C).
In a CRISPR region, these bases appear in the aforementioned order several times, and in these repeated segments, they course what's known equally "palindromic" sequences, according to the Max Planck Institute. A palindrome, like the word "racecar," reads the same forward as it does backward; similarly, in a palindromic sequence, bases on one side of the DNA ladder match those on the opposing side when you read them in opposite directions.
For example, a super simple palindromic sequence might expect like this:
- Side 1 - GATC
- Side 2 - CTAG
Brusk palindromic repeats appear throughout CRISPR regions of DNA, with each echo bookended by "spacers." Bacteria swipe such spacers from viruses that have attacked them, meaning they incorporate a bit of viral DNA into their own genome. These spacers serve as a bank of memories, which enables the bacteria to recognize the viruses if they should e'er assail again. You can also retrieve of spacers like "Wanted" posters, providing a snapshot of the bad guys so they can be easily spotted and brought to justice.
Related: Going viral: 6 new findings about viruses
Rodolphe Barrangou and a squad of researchers at Danisco, a food ingredients company, start demonstrated this procedure experimentally. In a 2007 newspaper published in the periodical Science, the researchers used Streptococcus thermophilus leaner, which are normally found in yogurt and other dairy cultures, as their model, co-ordinate to the Articulation Genome Constitute, part of the U.South. Department of Energy. They observed that later on a viral assail, the leaner incorporated new spacers into their CRISPR regions. Moreover, the DNA sequence of these spacers was identical to parts of the virus genome.
The squad likewise manipulated the spacers by removing them and inserting new viral DNA sequences in their place. In this fashion, the researchers were able to alter the bacteria's resistance to an attack by a specific virus, confirming CRISPRs' function in regulating bacterial immunity.
CRISPR RNA (crRNA) : CRISPR regions of Dna human activity every bit a kind of bank of viral memories; but for that stored information to be useful elsewhere in the cell, it must exist copied, or "transcribed," into a unlike genetic molecule chosen RNA. Dissimilar DNA sequences, which remain lodged within the DNA molecule, this CRISPR RNA (crRNA) can roam nigh the cell and squad upwards with proteins — namely the molecular scissors that snip viruses to bits.
RNA also differs from DNA in that it'due south only one strand, rather than two, significant it looks like but a one-half of a ladder. To build an RNA molecule, one role of the CRISPR acts as a template and proteins called polymerases dive in to construct an RNA molecule that is "complementary" to that template, pregnant the 2 strands' bases would fit together similar puzzle pieces. For example, a G in the DNA molecule would get transcribed every bit a C in the RNA.
Each snippet of CRISPR RNA contains a re-create of a repeat and a spacer from a CRISPR region of DNA, co-ordinate to a 2022 review by Jennifer Doudna and Emmanuelle Charpentier, published in the periodical Science. The crRNA interacts with the Cas9 protein and another kind of RNA, chosen "trans-activating crRNA" or tracrRNA, in order to help bacteria fend off viruses.
Cas9: The Cas9 poly peptide is an enzyme that cuts foreign Deoxyribonucleic acid. The protein binds to crRNA and tracrRNA, which together guide Cas9 to a target site on the virus's DNA strand where the poly peptide will make its cut. The target DNA that the Cas9 volition cut through is complementary to a 20-nucleotide stretch of the crRNA, where a "nucleotide" is a building block of DNA that contains one base of operations.
Using two dissever regions or "domains" on its structure, Cas9 cuts both strands of the DNA double helix, making what is known as a "double-stranded break," according to the 2022 Science commodity.
There is a built-in safety machinery that ensures that Cas9 doesn't just cut just anywhere in a genome. Short Dna sequences known as "protospacer adjacent motifs," or PAMs, serve every bit tags and sit adjacent to the target Dna sequence. If the Cas9 complex doesn't see a PAM next to its target DNA sequence, it won't cut. This is one possible reason that Cas9 doesn't always attack the CRISPR region in bacteria, according to a 2022 review published in Nature Biotechnology.
How CRISPR works as a genome-editing tool
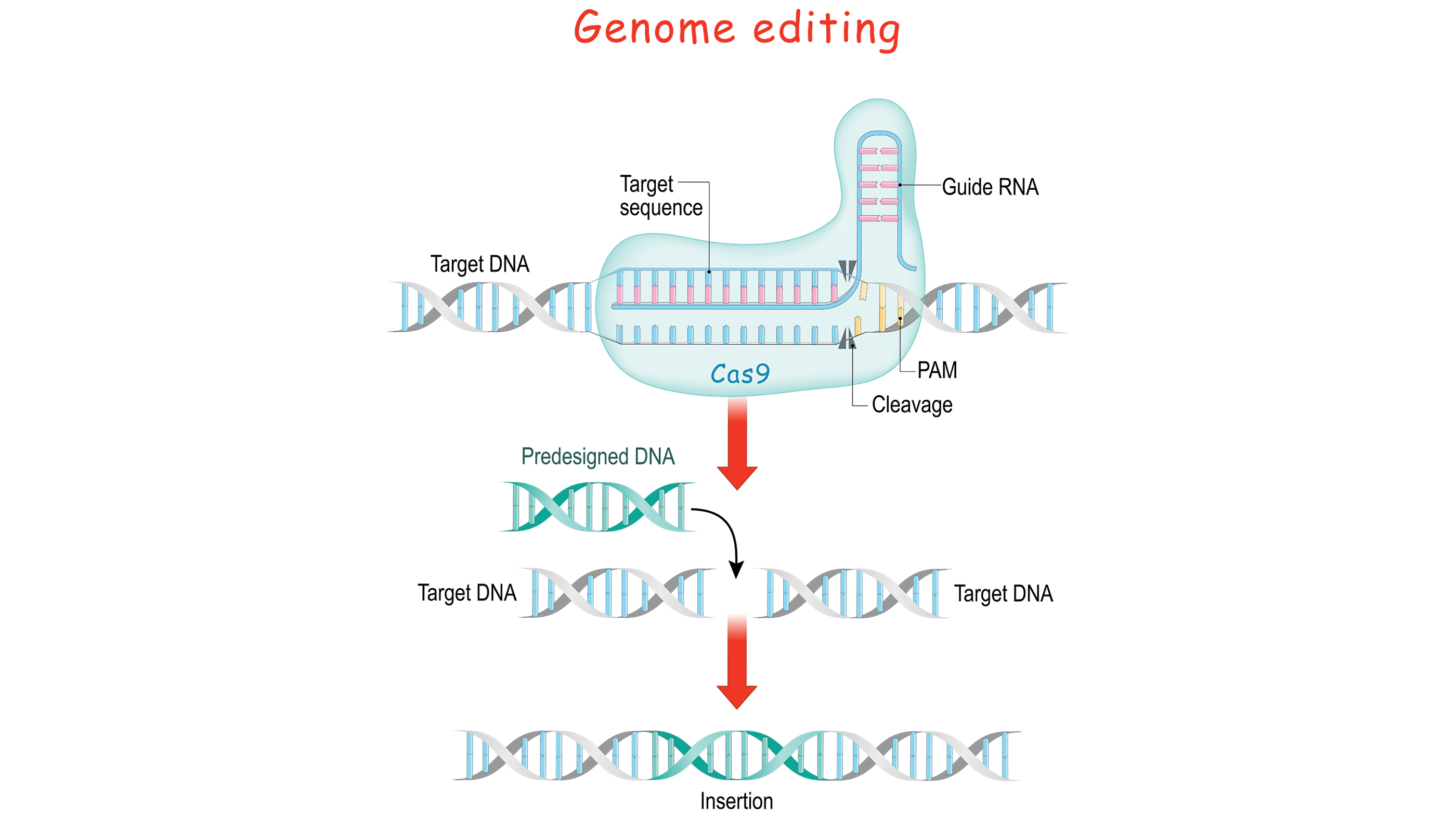
Genomes encode a serial of letters and instructions within their DNA sequences, and genome editing involves irresolute those sequences, thereby changing the messages they contain. This can be done by inserting a cutting or break in the DNA and tricking a jail cell'southward natural Dna repair mechanisms into introducing the targeted changes. CRISPR-Cas9 provides a means to do so.
In 2022, 2 pivotal research papers were published in the journals Science and PNAS, describing how the bacterial CRISPR-Cas9 could be used to chop up any DNA, not just that of viruses. In this style, the natural CRISPR organisation could be transformed into a unproblematic, programmable genome-editing tool.
To direct Cas9 to snip a specific region of DNA, scientists can simply alter the sequence of the crRNA, which binds to a complementary sequence in the target DNA, the studies ended.In the 2022 Science commodity, Martin Jinek and his colleagues further simplified the arrangement by fusing crRNA and tracrRNA to create a single "guide RNA." Thus, genome editing requires only two components: a guide RNA and the Cas9 poly peptide.
"Operationally, you design a stretch of xx base pairs that match a cistron that you want to edit," and from there, i tin figure out what the complementary crRNA sequence would be, George Church building, a professor of genetics at Harvard Medical School, told Live Science. Church emphasized the importance of making sure that the nucleotide sequence is found only in the target gene and nowhere else in the genome.
"Then the RNA plus the protein [Cas9] volition cutting — like a pair of scissors — the Deoxyribonucleic acid at that site, and ideally nowhere else," Church explained. Once the DNA is cut, the cell'southward natural repair mechanisms kick in and work to piece the DNA dorsum together, and at this bespeak, edits can be fabricated to the genome. There are 2 ways this can happen:
According to the Huntington's Outreach Projection at Stanford Academy, ane repair method involves gluing the two cuts back together. This method, known as "not-homologous end joining," tends to introduce errors where nucleotides are accidentally inserted or deleted, resulting in mutations that could disrupt a gene.
In the second method, the interruption is fixed past filling in the gap with a sequence of nucleotides. In lodge to exercise so, the cell uses a brusk strand of Deoxyribonucleic acid equally a template. Scientists can supply the Deoxyribonucleic acid template of their choosing, thereby writing-in whatsoever factor they want, or correcting a mutation.
Who discovered CRISPR?
Scientists originally discovered the CRISPRs in leaner in 1987, but they didn't initially sympathise the biological significance of the DNA sequences, and they didn't yet call them "CRISPRs," according to Quanta Mag. Yoshizumi Ishino and colleagues at Osaka University in Nihon first constitute the characteristic nucleotide repeats and spacers in the gut microbe Escherichia coli , and as the technology for genetic analysis improved in the 1990s, other researchers found CRISPRs in many other microbes.
Francisco Mojica, a scientist at the Academy of Alicante in Spain, was the first to describe the singled-out characteristics of CRISPRs and institute the sequences in 20 unlike microbes, according to a 2022 report in the journal Cell. At i indicate, he dubbed the sequences "short regularly spaced repeats" (SRSRs), but he after suggested that they be called CRISPRs instead. The term CRISPR first appeared in a 2002 study, published in the journal Molecular Microbiology and authored by Ruud Jansen of Utrecht University, with whom Mojica had been in correspondence.
In the following years, scientists also discovered Cas genes and the function of Cas enzymes, and they figured out that the spacers in CRISPRs came from invasive viruses, Quanta reported.
Amidst these pioneering researchers was Jennifer Doudna, a professor of biochemistry, biophysics and structural biological science at the University of California, Berkeley, who went on to share the 2022 Nobel Prize in chemistry with Emmanuelle Charpentier, managing director of the Max Planck Unit of measurement for the Science of Pathogens. The 2 scientists are credited with adapting the bacterial CRISPR/Cas system into a handy factor-editing tool, Live Science previously reported.
Related: Nobel Prize in Chemistry: 1901-Present
Charpentier initially discovered tracrRNA while studying the leaner Streptococcus pyogenes, which causes a range of diseases from tonsillitis to sepsis. Having uncovered tracrRNA every bit a previously unknown component of the CRISPR/Cas system, Charpentier began collaborating with Doudna to recreate that arrangement in a exam tube. In 2022, the squad published their seminal work in the journal Science, announcing that they'd successfully simplified the molecular scissors into a gene-editing tool.
Some thought that biochemist Feng Zhang of the Wide Institute might as well earn the Nobel for his own, divide work with the CRISPR system, Scientific discipline Magazine reported. Zhang demonstrated that the CRISPR system works in mammalian cells, and based on this piece of work, the Broad Institute earned the first patent for the employ of CRISPR gene-editing technology in eukaryotes, or complex cells with nuclei to hold their DNA.
How has CRISPR been used?
In 2022, researchers in the labs of Church and Zhang published the first reports describing the apply of CRISPR-Cas9 to edit human cells in an experimental setting. Studies conducted in lab dish and animal models of human disease have demonstrated that the engineering can finer right genetic defects. Examples of such diseases include cystic fibrosis, cataracts and Fanconi anemia, according to a 2022 review article published in the periodical Nature Biotechnology. These studies have paved the style for therapeutic applications in humans.
In the realm of medicine, CRISPR has been tested in early-phase clinical trials every bit cancer therapy and as a treatment for an inherited disorder that causes blindness. It's also been investigated as a strategy for preventing the spread of Lyme affliction and malaria from viral vectors to people, and it's likewise been studied in animal models of HIV as a mode to rid infected cells of the virus, Live Science previously reported. 1 research team in People's republic of china attempted to treat a human patient's HIV using CRISPR, and while the treatment wasn't successful in curing the infection, the factor therapy also didn't crusade any harmful effects, Live Science reported.
"I think the public perception of CRISPR is very focused on the idea of using cistron editing clinically to cure affliction," said Neville Sanjana of the New York Genome Center and an assistant professor of biology, neuroscience and physiology at New York University. "This is no doubt an exciting possibility, merely this is but one small slice."
Related: x amazing things scientists merely did with CRISPR
CRISPR technology has also been practical in the food and agricultural industries to engineer probiotic cultures and to vaccinate industrial cultures (yogurt, for instance) against viruses. It is besides existence used in crops to better yield, drought tolerance and nutritional properties.
One other potential application is to create factor drives, a genetic engineering technique that increases the chances of a particular trait passing on from parent to offspring; this kind of genetic engineering derives from a natural phenomenon, where specific versions of genes are more likely to be inherited. Somewhen, over the course of generations, the trait spreads through unabridged populations, according to the Wyss Institute. Gene drives could be used for various applications, such as eradicating invasive species or reversing pesticide and herbicide resistance in crops, according to a 2022 study published in the journal Scientific discipline.
During the COVID-19 pandemic, the CRISPR-Cas9 arrangement has been used to develop diverse diagnostic tests for the viral infection, BBC News reported.
In addition, CRISPR has recently been used in the following ways:
- In Apr 2022, a team of researchers released research in the periodical Science that they had programmed a CRISPR molecule to observe strains of viruses, such equally Zika, in blood serum, urine and saliva.
- On Aug. 2, 2022, scientists revealed in the journal Nature that they had removed a heart disease defect in an embryo successfully using CRISPR.
- On Jan. 2, 2022, researchers announced that they may be able to stop fungi and other problems that threaten chocolate production using CRISPR to brand the plants more than resistant to affliction.
- On Apr xvi, 2022, researchers upgraded CRISPR to edit thousands of genes at once, co-ordinate to inquiry published by the journal BioNews.
Yet, despite its broad range of uses, the tool is not without its drawbacks.
"I retrieve the biggest limitation of CRISPR is it is not a hundred percent efficient," Church told Alive Science. That means, in a given experiment, CRISPR may successfully edit merely a percentage of the targeted DNA. Co-ordinate to the 2022 Science article by Doudna and Charpentier, in a study conducted in rice, gene editing occurred in nigh 50% of the cells that received the Cas9-RNA complex. Meanwhile, other analyses take shown that depending on the target, editing efficiencies can reach as high as fourscore% or more.
The engineering can besides create "off-target effects" when Deoxyribonucleic acid is cut at sites other than the intended target. This can lead to the introduction of unintended mutations. Furthermore, Church noted, even when the arrangement cuts on target, there is a risk of not getting a precise edit. He chosen this "genome vandalism."
Potential risks and ethical concerns of using CRISPR
The many potential applications of CRISPR applied science raise questions nearly the ethical claim and consequences of tampering with genomes. And in particular, a slew of ethical debates flared up in 2022 when He Jiankui, formerly a biophysicist at the Southern University of Scientific discipline and Engineering science in Shenzhen, appear that his team had edited Dna in human being embryos and thus created the world's first gene-edited babies.
He was subsequently sentenced to iii years in prison and fined 3 million yuan ($560,000) for practicing medicine without a license, violating Chinese regulations on human-assisted reproductive technology and fabricating ethical review documents, Live Science previously reported. But even after his sentencing, He's experiments raised questions about how the apply of CRISPR should be regulated going forrad, especially given that the technology is still fairly new.
Related: Here'southward what nosotros know virtually CRISPR safety
Illegal experimentation in man embryos represents an extreme misuse of CRISPR, of course, but even seemingly ethical uses of the technology could carry risks, scientists say.
In full general, making genetic modifications to homo embryos and reproductive cells such as sperm and eggs is known as germline editing. Since changes to these cells can exist passed on to subsequent generations, using CRISPR technology to make germline edits has raised a number of ethical concerns.
Variable efficacy, off-target furnishings and imprecise edits all pose condom risks. In add-on, there is much that is withal unknown to the scientific community. In a 2022 article published in Scientific discipline, David Baltimore and a group of scientists, ethicists and legal experts annotation that germline editing raises the possibility of unintended consequences for future generations "considering there are limits to our knowledge of human genetics, gene-environment interactions, and the pathways of disease (including the interplay between ane disease and other atmospheric condition or diseases in the same patient)."
In the 2022 Scientific discipline commodity, Oye and colleagues betoken to the potential ecological bear on of using gene drives. An introduced trait could spread beyond the target population to other organisms through crossbreeding. Gene drives could as well reduce the genetic diversity of the target population, potentially hampering its ability to survive.
Other ethical concerns are more nuanced. Should we make changes that could fundamentally affect future generations without having their consent? What if the use of germline editing veers from existence a therapeutic tool to an enhancement tool for diverse human characteristics?
To address these concerns, the National Academies of Sciences, Engineering and Medicine put together a comprehensive report with guidelines and recommendations for genome editing.
Although the National Academies urge circumspection in pursuing germline editing, they emphasize "circumspection does non mean prohibition." They recommend that germline editing exist done only on genes that lead to serious diseases and just when at that place are no other reasonable treatment alternatives. Among other criteria, they stress the need to collect data on the health risks and benefits and to maintain continuous oversight during clinical trials. They also recommend that, later a trial concludes, trial organizers should follow up with the participants' families for multiple generations to come across what changes persist in the genome over fourth dimension.
Additional resources
- Watch this blitheness from TEDEd to larn how CRISPR lets scientists edit Deoxyribonucleic acid.
- Mind to Jennifer Doudna deliver her Nobel Lecture afterward winning the prize in 2022.
- Read about the ongoing boxing over CRISPR patents in Scientific discipline Magazine.
This article includes boosted reporting by Alina Bradford, Live Scientific discipline contributor.
Originally published on Live Scientific discipline.
Source: https://www.livescience.com/58790-crispr-explained.html
Posted by: gibbonshising.blogspot.com



0 Response to "How Is The Template Strand Determined In Repair"
Post a Comment